
Adaptive Radiotherapy in Focus
Trials and Real-World Impact
Adaptive Radiotherapy is rapidly evolving from a research concept to standard clinical practice, offering precise, patient-tailored radiation delivery. By combining real-time imaging, daily plan adaption, and advanced workflow tools, Adaptive Radiotherapy helps clinicians maximize tumor control while minimizing toxicity. These innovations are reshaping the standard of care, providing both measurable clinical benefits and practical insights for optimizing treatment across multiple cancer types.
Compare Imaging TechnologiesAdaptive Radiotherapy in Action
Treatment-Changing Clinical Evidence
Key clinical trials highlight Adaptive Radiotherapy’s feasibility, safety, and efficacy across tumor sites.
| Cancer Type | Trial / Study | Sponsor / Institution | Highlights / Outcomes |
|---|---|---|---|
| Brain (High-Grade Glioma) | UNITED (NCT04726397) & UNITED-3 (NCT05720078) | Sunnybrook Health Sciences Centre | Demonstrated feasibility of large margin reductions (40–71%) with MR-Linac Adaptive Radiotherapy. UNITED-3 expands to two-phase adaptive therapy for glioblastoma.[3] |
| Gynecologic (Cervical) | EMBRACE I & II | Vienna/International Consortium | EMBRACE I: ~92% 5-year local control with low morbidity using MRI-guided brachytherapy. EMBRACE II: protocol refinement with improved survival and reduced toxicity.[1][2] |
| Head & Neck | MSK Trial (NCT03096808) | Memorial Sloan Kettering | Phase II trial testing daily Adaptive Radiotherapy in anatomically complex head & neck cancers, assessing feasibility and toxicity.[8] |
| Liver | MRI-Guided SBRT (NCT02683200) | Jonsson Comprehensive Cancer Center | Evaluates Adaptive Radiotherapy in treating primary/metastatic liver tumors, addressing challenges of tumor motion and deformation.[9] |
| Multi-Cancer (Registry) | MOMENTUM (NCT04075305) | MR-Linac Consortium | Global, multi-institutional registry collecting prospective Adaptive Radiotherapy outcomes, building a large-scale evidence base.[10] |
| Pancreas | SMART (NCT03621644) | ViewRay Inc. | Phase II MR-guided Adaptive Radiotherapy trial showed favorable 2-year overall survival (~40–54%), high local control (78%), and minimal severe GI toxicity, supporting its safety and efficacy.[11] |
| Prostate | HERMES (NCT04595019) | Royal Marsden Hospital, London | Compared 2- vs. 5-fraction MR-guided Adaptive Radiotherapy schedules. Interim data show favorable toxicity profile in ultra-hypofractionated schedules.[4] |
| Prostate | DESTINATION 2 (NCT06638541) | MR-Linac Consortium | Investigating fractionation and workflow optimization in Adaptive Radiotherapy for prostate cancer.[5] |
| Prostate | ASPIRE (NCT06825091) | University Health Network, Toronto | MR-guided adaptive SBRT in localized prostate cancer, aiming to improve urinary outcomes while maintaining cancer control compared to conventional CBCT-guided SBRT..[6] |
| Rectal | MARS | MR-Linac Consortium | Exploring Adaptive Radiotherapy strategies in rectal cancer with hypofractionated schedules.[7] |
| Rectal | Adaptive Boost Trial (NCT06246344) | Shandong Cancer Hospital and Institute | Phase III trial assessing the efficacy and safety of MR/CBCT/FBCT-guided adaptive boost radiotherapy in locally advanced rectal cancer.[12] |
Bridging the Gap
From Evidence to Everyday Adaptive Radiotherapy
Alongside controlled trials, real-world studies are accelerating Adaptive Radiotherapy adoption.
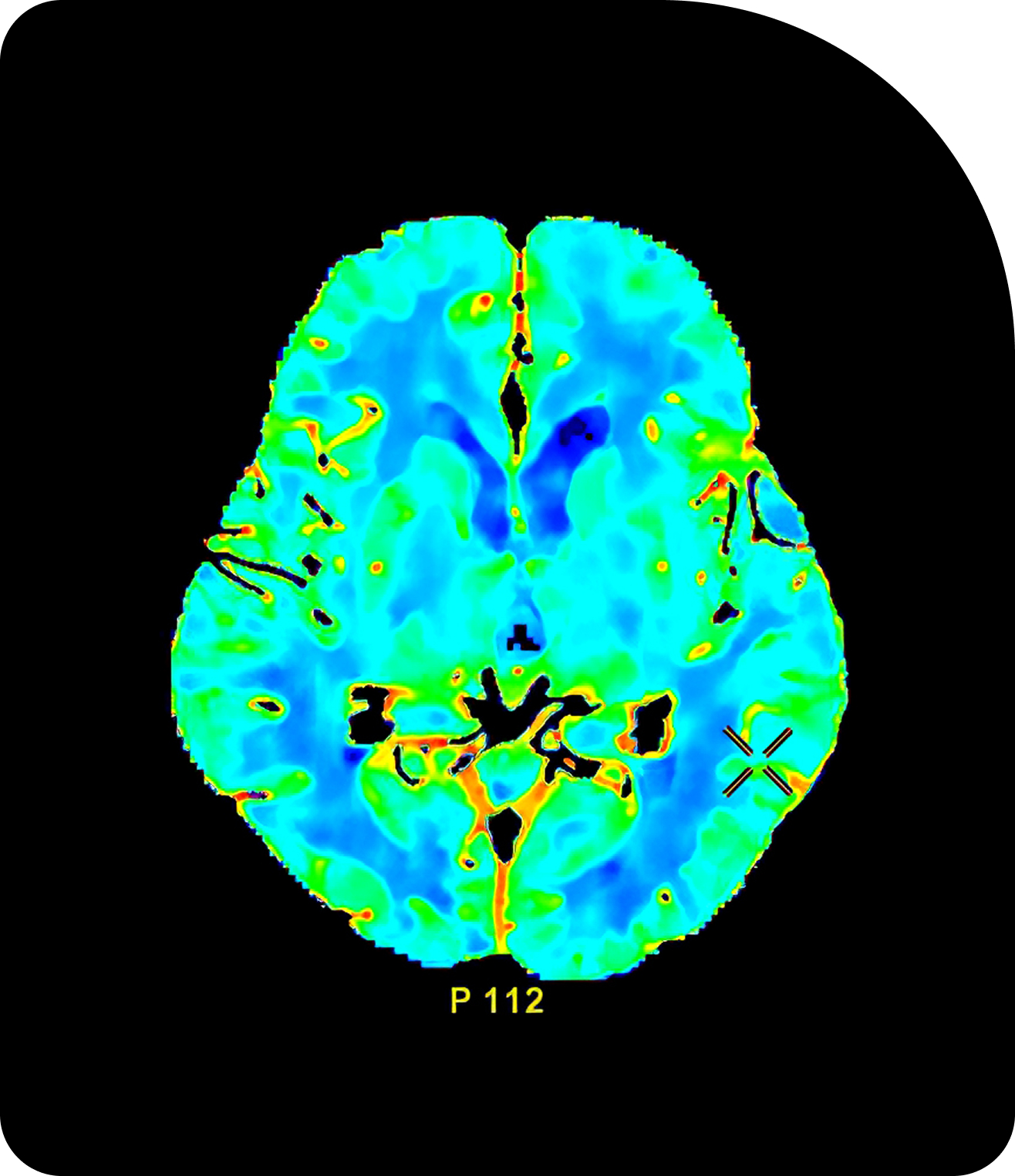
Digital twin simulations
Validate prostate SBRT plans under variable anatomy, ensuring treatment consistency across patients.
AI-driven segmentation models
Including CT and MR auto-segmentation, streamlines Adaptive Radiotherapy workflows in cancers such as esophageal, head & neck, prostate, pancreatic and lung, reducing planning time while maintaining precision and treatment accuracy.
Imaging-based triage approaches
In cervical cancer help identify patients most likely to benefit from adaption, allowing personalized workflow prioritization.
These studies demonstrate Adaptive Radiotherapy’s practical integration into clinical practice, ensuring that advanced technologies improve efficiency and maintain high standards of patient safety.
The Road Ahead for Adaptive Radiotherapy
The evidence base to date underscores Adaptive Radiotherapy’s transformative potential in multiple disease sites. Across gynecologic, brain, prostate, liver, and head & neck cancers, Adaptive Radiotherapy consistently enables personalized treatment, improved local control, and reduced toxicity.
As these studies mature, Adaptive Radiotherapy is poised to broaden access to safer, more effective, and more precise care for patients worldwide, establishing a new standard in radiation oncology that balances innovation with tangible clinical benefit.

Ready to Get Started?
Contact usReferences
- Pötter R, et al. EMBRACE I: Local control and morbidity after MRI-guided adaptive brachytherapy in cervical cancer. Lancet Oncol. 2018.
- Sturdza A, et al. EMBRACE II: Refining image-guided brachytherapy protocols. Radiother Oncol. 2021.
- National Brain Tumor Society. UNITED & UNITED-3 trials overview. Accessed 2025.
- Murray J, et al. HERMES: Randomized Trial of 2-Fraction or 5-Fraction Magnetic Resonance Imaging–Guided Adaptive Prostate Radiation Therapy. International Journal of Radiation Oncology, Biology, Physics. 2025.
- MR-Linac Consortium. DESTINATION 2 Hypothesis Testing Program. Accessed 2025.
- ClinicalTrials.gov ID NCT06825091. Is Adaptive SBRT for Prostate vs Image-guided Radiotherapy a True Evolution (ASPIRE). University Health Network. Updated 2025.
- MR-Linac Consortium. MARS rectal cancer trial. Accessed 2025.
- ClinicalTrials.gov ID NCT03096808. Adaptive Radiotherapy for Head and Neck Cancer. Memorial Sloan Kettering. Updated 2021.
- ClinicalTrials.gov ID NCT02683200. MRI-Guided SBRT for Liver Cancer. Jonsson Comprehensive Cancer Center. Updated 2023.
- MR-Linac Consortium. MOMENTUM Registry. Accessed 2025.
- Chuong, Michael D. et al. Stereotactic MR-guided on-table adaptive radiation therapy (SMART) for borderline resectable and locally advanced pancreatic cancer: A multi-center, open-label phase 2 study. Radiotherapy and Oncology. 2023.
- ClinicalTrials.gov ID NCT06246344. Adaptive Boost Radiotherapy to Primary Lesions and Positive Nodes in the Neoadjuvant Treatment of Locally Advanced Rectal Cancer. Shandong Cancer Hospital and Institute. Last updated 2025.

